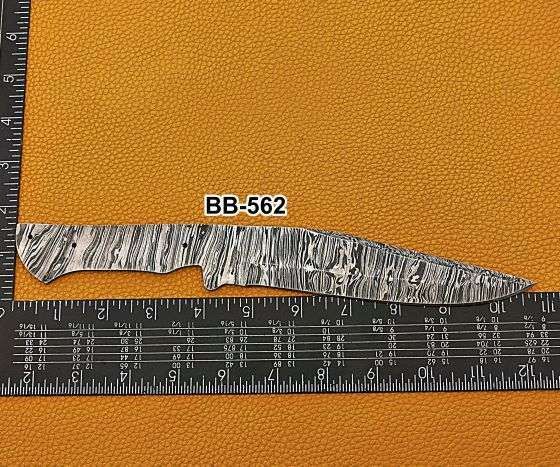
Description
10.75 inches long Kukri point Blank blade, hand forged Ladder pattern Damascus steel hunting knife blade, knife making supplies, 3 Pin hole scale, 6″ sharp cutting edge
Damascus steel hunting
Twist Pattern Damascus steel Kukri point blade
Blade is made of Damascus steel by forging the 1095 and 15N20 high and low carbon steel Our Damascus steel is prepared by forging over 465 layer of high carbon and low carbon steel. Blade has been given an excellent heat treatment to get it well hardened. Hardness 56 to 58 HRC. Tips to Care Damascus knife: Damascus steel as well as 1095 high carbon steel knives are different than some other common steel knives because of its high carbon content they can be rusted if not care properly. If you have seen rust accidentally then use WD40 to remove it. Never store your knife for long time in leather sheath. Leather can absorb water which will rust the knife. Always clean the blade after using and apply oil or wax (please use cooking oil on Chef Knife) before you store it, for its longer life and durability – Damascus steel hunting
Steel is an alloy made up of iron with typically a few tenths of a percent of carbon to improve its strength and fracture resistance compared to other forms of iron. Many other elements may be present or added. Stainless steels that are corrosion- and oxidation-resistant need typically an additional 11% chromium.
Because of its high tensile strength and low cost, steel is used in buildings, infrastructure, tools, ships, trains, cars, machines, electrical appliances, and weapons. Iron is the base metal of steel. Depending on the temperature, it can take two crystalline forms (allotropic forms): body centred cubic and face centred cubic. The interaction of the allotropes of iron with the alloying elements, primarily carbon, gives steel and cast iron their range of unique properties – Damascus steel hunting
In pure iron, the crystal structure has relatively little resistance to the iron atoms slipping past one another, and so pure iron is quite ductile, or soft and easily formed. In steel, small amounts of carbon, other elements, and inclusions within the iron act as hardening agents that prevent the movement of dislocations.
The carbon in typical steel alloys may contribute up to 2.14% of its weight.[1] Varying the amount of carbon and many other alloying elements, as well as controlling their chemical and physical makeup in the final steel (either as solute elements, or as precipitated phases), impedes the movement of the dislocations that make pure iron ductile, and thus controls and enhances its qualities.
These qualities include the hardness, quenching behaviour, need for annealing, tempering behaviour, yield strength, and tensile strength of the resulting steel. The increase in steel’s strength compared to pure iron is possible only by reducing iron’s ductility – Damascus steel hunting
Steel was produced in bloomery furnaces for thousands of years, but its large-scale, industrial use began only after more efficient production methods