Description
20 pieces Lot of 7 inches long Damascus steel back lock folding knife with s Blue and white Texas handle, Comes with Sheath
Tips to Care Damascus knife:
Damascus steel as well as 1095 high carbon steel knives are different than some other common steel knives, because of its high carbon content they can be rusted if not care properly. If you have seen rust accidently then use WD40 to remove it.
Never store your knife for long time in leather sheath. Leather can absorb water which will rust the knife. Always clean the blade after using and apply oil or wax (please use cooking oil on Chef Knife) before you store it, for its longer life and durability – Damascus steel pocket clip
DISCLAIMER:
Our knives are very sharp so open and use them very carefully.
We are not responsible for any injuries associated with the use of our knives.
Our products are intended for legal use only by responsible buyers.
We will not sell our products to anyone under the age of 18.
We will not responsible for custom held in your country (for international purchases).
We will not be responsible for shipping loss if addressed wrong – Damascus steel pocket clip
Steel is an alloy made up of iron with typically a few tenths of a percent of carbon to improve its strength and fracture resistance compared to other forms of iron. Many other elements may be present or added. Stainless steels that are corrosion- and oxidation-resistant need typically an additional 11% chromium.
Because of its high tensile strength and low cost, steel is used in buildings, infrastructure, tools, ships, trains, cars, machines, electrical appliances, and weapons. Iron is the base metal of steel. Depending on the temperature, it can take two crystalline forms (allotropic forms): body centred cubic and face centred cubic. The interaction of the allotropes of iron with the alloying elements, primarily carbon, gives steel and cast iron their range of unique properties.
In pure iron, the crystal structure has relatively little resistance to the iron atoms slipping past one another, and so pure iron is quite ductile, or soft and easily formed. In steel, small amounts of carbon, other elements, and inclusions within the iron act as hardening agents that prevent the movement of dislocations.
The carbon in typical steel alloys may contribute up to 2.14% of its weight.[1] Varying the amount of carbon and many other alloying elements, as well as controlling their chemical and physical makeup in the final steel (either as solute elements, or as precipitated phases), impedes the movement of the dislocations that make pure iron ductile, and thus controls and enhances its qualities.
These qualities include the hardness, quenching behaviour, need for annealing, tempering behaviour, yield strength, and tensile strength of the resulting steel. The increase in steel’s strength compared to pure iron is possible only by reducing iron’s ductility.



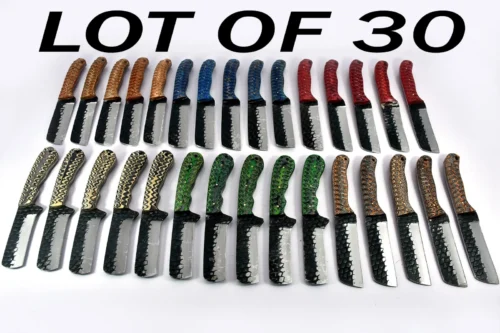























 7 Inch Long Hand forged Damascus steel folding knife, Natural Bull Horn scale with Damascus bolster, Equipped with Liner lock and thumb knob, Cow hide Leather sheath (Wood)
7 Inch Long Hand forged Damascus steel folding knife, Natural Bull Horn scale with Damascus bolster, Equipped with Liner lock and thumb knob, Cow hide Leather sheath (Wood)